Acetone is treated with excess of ethanol in the presence of hydrochloric acid. The product obtained is:

Explain the reaction between acetone and ethanol - Chemistry - Solutions - 12698165 | Meritnation.com
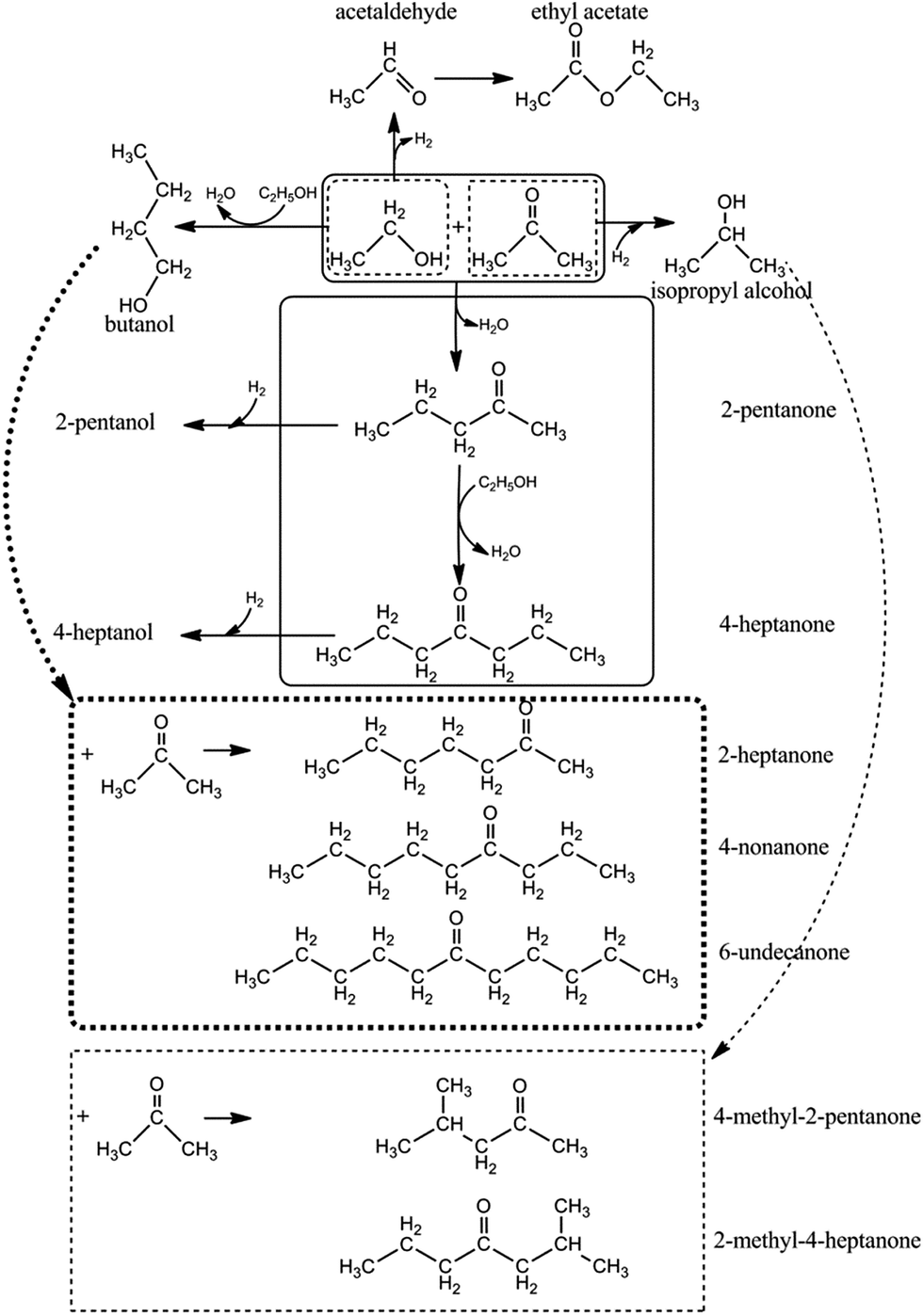
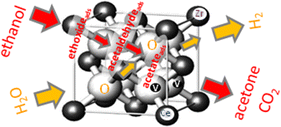
Acetone alkylation with ethanol over multifunctional catalysts by a borrowing hydrogen strategy - RSC Advances (RSC Publishing) DOI:10.1039/C5RA17889D

thermodynamics - What mixing ratio of ethanol and acetone has the lowest freezing point? - Chemistry Stack Exchange

Structure formula and size of acetone, ethanol, and water, considering... | Download Scientific Diagram

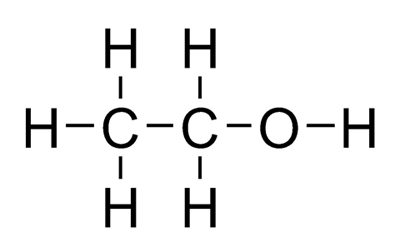
Draw the structures of ethanol, acetone, toluene, hexane, and water. Classify each solvent as polar, nonpolar, or moderately polar. | Homework.Study.com

solutions - What are the intermolecular forces between the following compounds in a mixture? - Chemistry Stack Exchange